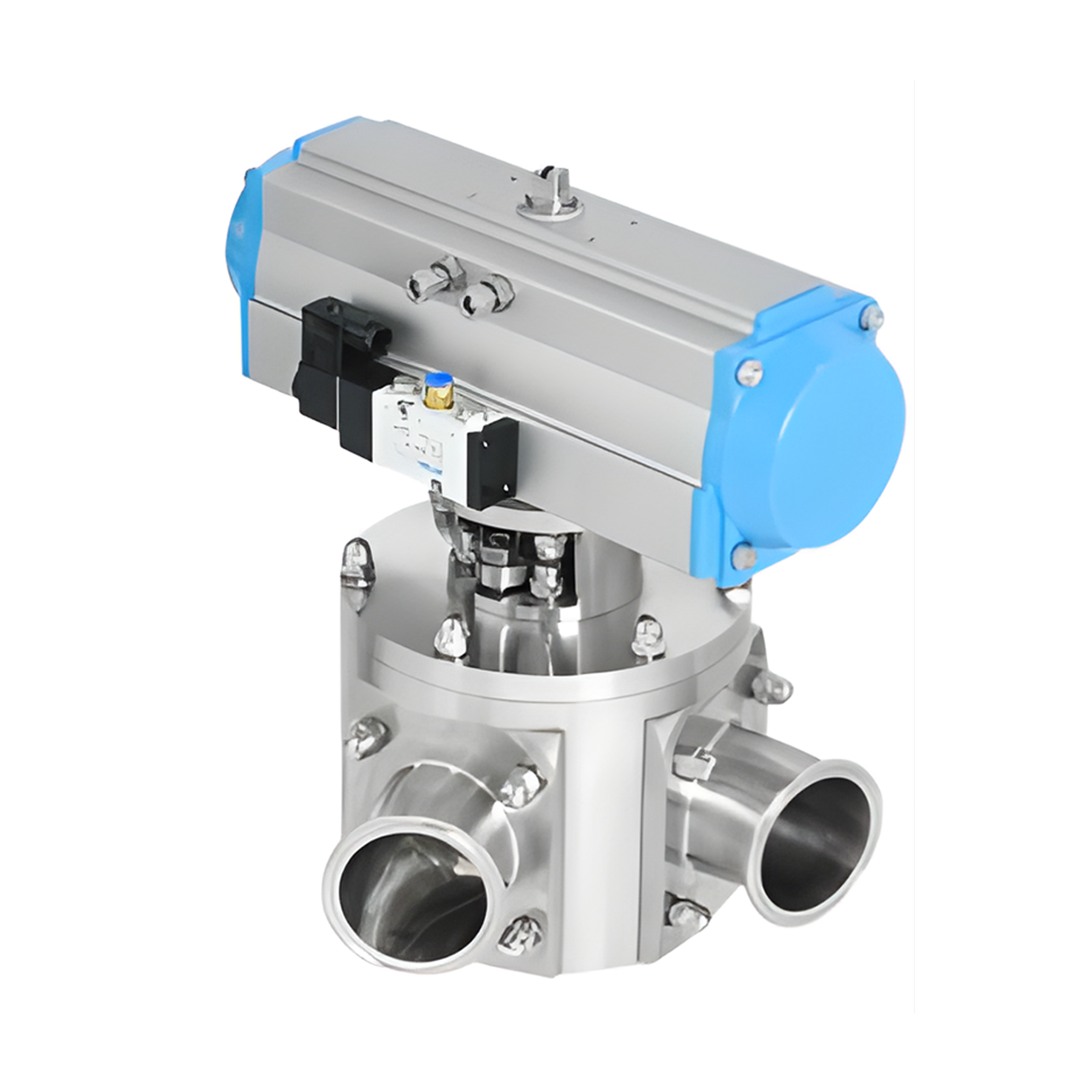
Ακριβής Δειγματοληψία Υλικού με Βαλβίδες Δειγματοληψίας
Η βαλβίδα δειγματοληψίας προορίζεται για τη συλλογή αντιπροσωπευτικών δειγμάτων από σωληνώσεις ή δοχεία, εξασφαλίζοντας ότι το δείγμα αντικατοπτρίζει την πραγματική σύνθεση του υλικού εντός. Χρησιμοποιείται συχνά στις χημικές, τροφιμικές και φαρμακευτικές βιομηχανίες, επιτρέποντας ακριβή δειγματοληψία για έλεγχο ποιότητας και δοκιμές. Σχεδιασμένες για ελάχιστη διαταραχή της διαδικασίας, κατασκευάζονται για αξιοπιστία και εύκολη συντήρηση, διασφαλίζοντας ότι τα πρότυπα ποιότητας τηρούνται συνεχώς σε κρίσιμα περιβάλλοντα παραγωγής.
Λάβετε προσφορά